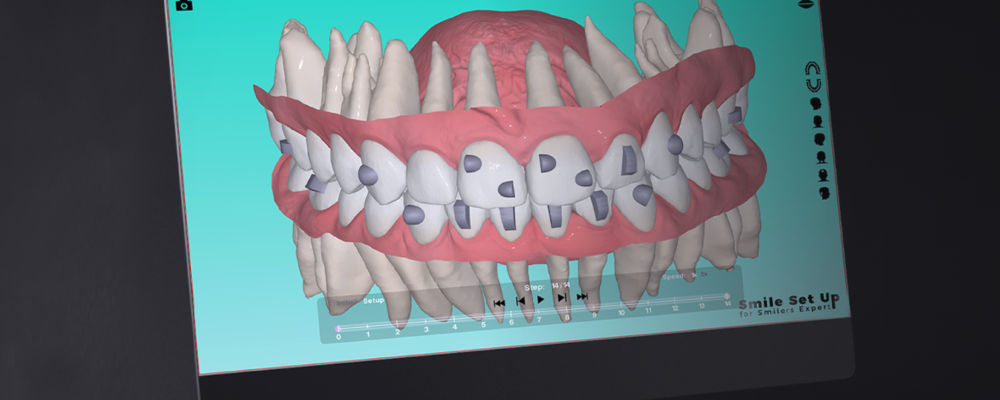
Biotech Dental est un concepteur et fabricant de dispositifs médicaux et de solutions numériques
à destination des chirurgiens-dentistes, des orthodontistes et des laboratoires de prothèse dentaire.
La mission de notre groupe est de donner les moyens aux professionnels du monde dentaire de prodiguer
des soins d’excellence grâce à des technologies innovantes.